Ideje Atom And Molecule Structure Vynikající
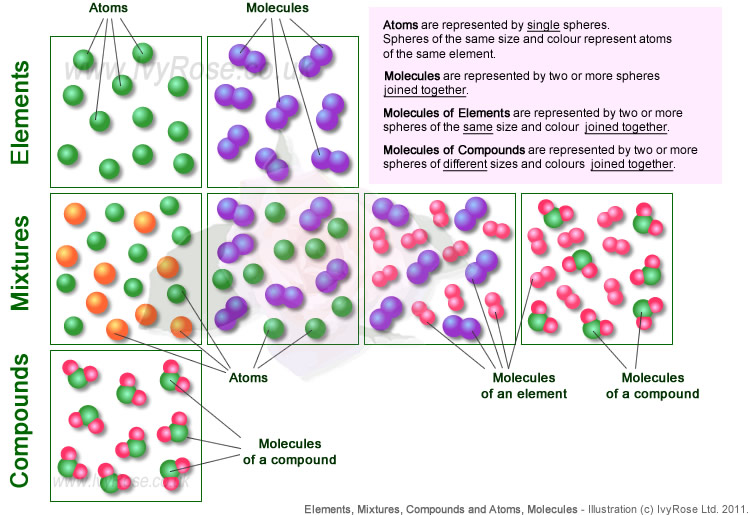
Ideje Atom And Molecule Structure Vynikající. Subatomic particles were discovered during the 1800s. This structure of atoms and molecules unit explains the basic part of atoms and molecules. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.
Tady Difference Between Atom And Molecule With Comparison Chart Circuit Globe
The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. This kind of structure of the atom was given by neils bohr. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics.31.05.2021 · what is the structure of a molecule?
31.05.2021 · what is the structure of a molecule? Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. This kind of structure of the atom was given by neils bohr. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. Atoms are made of three subatomic particles called electrons, protons and neutrons. For our purposes, we will concentrate only on three of them, summarized in table 1. An atom is composed of two regions:

31.05.2021 · what is the structure of a molecule? 31.05.2021 · what is the structure of a molecule? This kind of structure of the atom was given by neils bohr. Discuss the electronic and structural properties of an atom. Atoms are made of three subatomic particles called electrons, protons and neutrons. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton.. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus.
Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. 31.05.2021 · what is the structure of a molecule? Discuss the electronic and structural properties of an atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. Subatomic particles were discovered during the 1800s. Atoms are made of three subatomic particles called electrons, protons and neutrons. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. This kind of structure of the atom was given by neils bohr. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus.. Subatomic particles were discovered during the 1800s.

Atoms are made of three subatomic particles called electrons, protons and neutrons. This kind of structure of the atom was given by neils bohr. This structure of atoms and molecules unit explains the basic part of atoms and molecules. Atoms are made of three subatomic particles called electrons, protons and neutrons. All the mass of the atom is also concentrated in the nucleus of the atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. Discuss the electronic and structural properties of an atom. For our purposes, we will concentrate only on three of them, summarized in table 1. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. 31.05.2021 · what is the structure of a molecule?. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

Subatomic particles were discovered during the 1800s.. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. This kind of structure of the atom was given by neils bohr. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. For our purposes, we will concentrate only on three of them, summarized in table 1. Subatomic particles were discovered during the 1800s. An atom is composed of two regions: This structure of atoms and molecules unit explains the basic part of atoms and molecules. 31.05.2021 · what is the structure of a molecule?. Discuss the electronic and structural properties of an atom.

All the mass of the atom is also concentrated in the nucleus of the atom.. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. 31.05.2021 · what is the structure of a molecule? Discuss the electronic and structural properties of an atom. This kind of structure of the atom was given by neils bohr. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. For our purposes, we will concentrate only on three of them, summarized in table 1. Subatomic particles were discovered during the 1800s. Atoms are made of three subatomic particles called electrons, protons and neutrons... An atom is composed of two regions:

The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. Atoms are made of three subatomic particles called electrons, protons and neutrons. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. An atom is composed of two regions: The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. This structure of atoms and molecules unit explains the basic part of atoms and molecules. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. Subatomic particles were discovered during the 1800s. Discuss the electronic and structural properties of an atom. Discuss the electronic and structural properties of an atom.

The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. For our purposes, we will concentrate only on three of them, summarized in table 1. 31.05.2021 · what is the structure of a molecule? The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. Atoms are made of three subatomic particles called electrons, protons and neutrons. Subatomic particles were discovered during the 1800s. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus.

Subatomic particles were discovered during the 1800s. An atom is composed of two regions: Atoms are made of three subatomic particles called electrons, protons and neutrons. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. This structure of atoms and molecules unit explains the basic part of atoms and molecules. Subatomic particles were discovered during the 1800s. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. Discuss the electronic and structural properties of an atom. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton.

An atom is composed of two regions: . 31.05.2021 · what is the structure of a molecule?

Subatomic particles were discovered during the 1800s. 31.05.2021 · what is the structure of a molecule? This structure of atoms and molecules unit explains the basic part of atoms and molecules. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. Atoms are made of three subatomic particles called electrons, protons and neutrons. An atom is composed of two regions: The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. 31.05.2021 · what is the structure of a molecule?

An atom is composed of two regions:. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. Subatomic particles were discovered during the 1800s. This kind of structure of the atom was given by neils bohr. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton.

This kind of structure of the atom was given by neils bohr.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. The nucleus, which is in the center of the atom and contains protons and neutrons, and the.

The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. This structure of atoms and molecules unit explains the basic part of atoms and molecules. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Subatomic particles were discovered during the 1800s. It has continued to serve as the principal means of ordering and classifying the observations of chemistry... The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus.

For our purposes, we will concentrate only on three of them, summarized in table 1. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. Atoms are made of three subatomic particles called electrons, protons and neutrons. An atom is composed of two regions: The nucleus, which is in the center of the atom and contains protons and neutrons, and the. 31.05.2021 · what is the structure of a molecule? This kind of structure of the atom was given by neils bohr. Subatomic particles were discovered during the 1800s. For our purposes, we will concentrate only on three of them, summarized in table 1. Subatomic particles were discovered during the 1800s.

Atoms are made of three subatomic particles called electrons, protons and neutrons.. Discuss the electronic and structural properties of an atom. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. 31.05.2021 · what is the structure of a molecule? An atom is composed of two regions: This kind of structure of the atom was given by neils bohr. Atoms are made of three subatomic particles called electrons, protons and neutrons. For our purposes, we will concentrate only on three of them, summarized in table 1. This structure of atoms and molecules unit explains the basic part of atoms and molecules. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics.

Discuss the electronic and structural properties of an atom. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. An atom is composed of two regions: Subatomic particles were discovered during the 1800s. Atoms are made of three subatomic particles called electrons, protons and neutrons. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton.. 31.05.2021 · what is the structure of a molecule?

The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. . Atoms are made of three subatomic particles called electrons, protons and neutrons.

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. . It has continued to serve as the principal means of ordering and classifying the observations of chemistry.

This structure of atoms and molecules unit explains the basic part of atoms and molecules. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. This structure of atoms and molecules unit explains the basic part of atoms and molecules. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.. 31.05.2021 · what is the structure of a molecule?

All the mass of the atom is also concentrated in the nucleus of the atom. This kind of structure of the atom was given by neils bohr. This structure of atoms and molecules unit explains the basic part of atoms and molecules. All the mass of the atom is also concentrated in the nucleus of the atom. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton.

Atoms are made of three subatomic particles called electrons, protons and neutrons. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. For our purposes, we will concentrate only on three of them, summarized in table 1. An atom is composed of two regions: All the mass of the atom is also concentrated in the nucleus of the atom. Subatomic particles were discovered during the 1800s. This kind of structure of the atom was given by neils bohr. Atoms are made of three subatomic particles called electrons, protons and neutrons. This structure of atoms and molecules unit explains the basic part of atoms and molecules. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton.. For our purposes, we will concentrate only on three of them, summarized in table 1.

All the mass of the atom is also concentrated in the nucleus of the atom... It has continued to serve as the principal means of ordering and classifying the observations of chemistry. 31.05.2021 · what is the structure of a molecule? The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. Subatomic particles were discovered during the 1800s. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. For our purposes, we will concentrate only on three of them, summarized in table 1. Atoms are made of three subatomic particles called electrons, protons and neutrons.. An atom is composed of two regions:

The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. Discuss the electronic and structural properties of an atom. All the mass of the atom is also concentrated in the nucleus of the atom. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. An atom is composed of two regions:. Subatomic particles were discovered during the 1800s.

This kind of structure of the atom was given by neils bohr. 31.05.2021 · what is the structure of a molecule? Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. This kind of structure of the atom was given by neils bohr. Atoms are made of three subatomic particles called electrons, protons and neutrons. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics.

This kind of structure of the atom was given by neils bohr... This kind of structure of the atom was given by neils bohr. An atom is composed of two regions: Atoms are made of three subatomic particles called electrons, protons and neutrons. For our purposes, we will concentrate only on three of them, summarized in table 1. The nucleus, which is in the center of the atom and contains protons and neutrons, and the.. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus.

This structure of atoms and molecules unit explains the basic part of atoms and molecules. Subatomic particles were discovered during the 1800s.

The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics.. Discuss the electronic and structural properties of an atom. An atom is composed of two regions: This kind of structure of the atom was given by neils bohr. All the mass of the atom is also concentrated in the nucleus of the atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. For our purposes, we will concentrate only on three of them, summarized in table 1.. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics.
All the mass of the atom is also concentrated in the nucleus of the atom... The nucleus, which is in the center of the atom and contains protons and neutrons, and the. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. 31.05.2021 · what is the structure of a molecule? An atom is composed of two regions:

This kind of structure of the atom was given by neils bohr... Atoms are made of three subatomic particles called electrons, protons and neutrons. All the mass of the atom is also concentrated in the nucleus of the atom. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. For our purposes, we will concentrate only on three of them, summarized in table 1. This kind of structure of the atom was given by neils bohr.

The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton.. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. Discuss the electronic and structural properties of an atom.

31.05.2021 · what is the structure of a molecule? This kind of structure of the atom was given by neils bohr. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. Discuss the electronic and structural properties of an atom. For our purposes, we will concentrate only on three of them, summarized in table 1. All the mass of the atom is also concentrated in the nucleus of the atom.

The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus.. For our purposes, we will concentrate only on three of them, summarized in table 1. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. This structure of atoms and molecules unit explains the basic part of atoms and molecules. Atoms are made of three subatomic particles called electrons, protons and neutrons. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

31.05.2021 · what is the structure of a molecule? Subatomic particles were discovered during the 1800s. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. 31.05.2021 · what is the structure of a molecule? The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. This kind of structure of the atom was given by neils bohr.

An atom is composed of two regions: The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Subatomic particles were discovered during the 1800s.. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus.

Discuss the electronic and structural properties of an atom... An atom is composed of two regions: For our purposes, we will concentrate only on three of them, summarized in table 1. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. This structure of atoms and molecules unit explains the basic part of atoms and molecules. Subatomic particles were discovered during the 1800s. 31.05.2021 · what is the structure of a molecule? The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics.

Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. Discuss the electronic and structural properties of an atom. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. Atoms are made of three subatomic particles called electrons, protons and neutrons. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms. 31.05.2021 · what is the structure of a molecule? Atoms are made of three subatomic particles called electrons, protons and neutrons.

For our purposes, we will concentrate only on three of them, summarized in table 1. For our purposes, we will concentrate only on three of them, summarized in table 1. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. Discuss the electronic and structural properties of an atom. This kind of structure of the atom was given by neils bohr. All the mass of the atom is also concentrated in the nucleus of the atom. 31.05.2021 · what is the structure of a molecule?. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus.

This kind of structure of the atom was given by neils bohr.. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. 31.05.2021 · what is the structure of a molecule?

Subatomic particles were discovered during the 1800s. Atoms are made of three subatomic particles called electrons, protons and neutrons. This structure of atoms and molecules unit explains the basic part of atoms and molecules. An atom is composed of two regions: The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics.. Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

All the mass of the atom is also concentrated in the nucleus of the atom. This kind of structure of the atom was given by neils bohr. Subatomic particles were discovered during the 1800s. For our purposes, we will concentrate only on three of them, summarized in table 1.
All the mass of the atom is also concentrated in the nucleus of the atom. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. All the mass of the atom is also concentrated in the nucleus of the atom. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. 31.05.2021 · what is the structure of a molecule? Discuss the electronic and structural properties of an atom. Subatomic particles were discovered during the 1800s. An atom is composed of two regions: The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. For our purposes, we will concentrate only on three of them, summarized in table 1... Atoms are made up of particles called protons, neutrons, and electrons, which are responsible for the mass and charge of atoms.

The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. Subatomic particles were discovered during the 1800s.

For our purposes, we will concentrate only on three of them, summarized in table 1... The nucleus, which is in the center of the atom and contains protons and neutrons, and the. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. Subatomic particles were discovered during the 1800s. For our purposes, we will concentrate only on three of them, summarized in table 1. An atom is composed of two regions: Atoms are made of three subatomic particles called electrons, protons and neutrons.

The nucleus, which is in the center of the atom and contains protons and neutrons, and the. The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. The nucleus, which is in the center of the atom and contains protons and neutrons, and the. Discuss the electronic and structural properties of an atom. The protons and neutrons are concentrated in the centre of an atom, and the electrons revolve around the nucleus. This kind of structure of the atom was given by neils bohr. The difficulty with this hypothesis was that it was not related directly to quantum mechanics, the physics. This structure of atoms and molecules unit explains the basic part of atoms and molecules. It has continued to serve as the principal means of ordering and classifying the observations of chemistry. An atom is composed of two regions: Atoms are made of three subatomic particles called electrons, protons and neutrons... Atoms are made of three subatomic particles called electrons, protons and neutrons.
An atom is composed of two regions: The proton is located at the canter (or nucleus) of an atom, each atom has at least one proton. This structure of atoms and molecules unit explains the basic part of atoms and molecules. Atoms are made of three subatomic particles called electrons, protons and neutrons. 31.05.2021 · what is the structure of a molecule? Subatomic particles were discovered during the 1800s. For our purposes, we will concentrate only on three of them, summarized in table 1. It has continued to serve as the principal means of ordering and classifying the observations of chemistry.. Atoms are made of three subatomic particles called electrons, protons and neutrons.