Seznamy Free Radical Atom
Seznamy Free Radical Atom. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Valence electrons are the electrons that are furthest away from the …
Tady Non Ionic Chemical Reactions
Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the … The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 21.01.2010 · free radicals are atoms that contain an unpaired electron. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.
A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15.07.2014 · free radicals are the products of normal cellular metabolism. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. In a homolytic fission, the bond breaks in such a way.

There are two types of bond fissions. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

This damage may play a role in the development of cancer and other. There are two types of bond fissions. Valence electrons are the electrons that are furthest away from the … A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. In a homolytic fission, the bond breaks in such a way... 21.01.2010 · free radicals are atoms that contain an unpaired electron.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... 21.01.2010 · free radicals are atoms that contain an unpaired electron. In a homolytic fission, the bond breaks in such a way. Homolytic and heterolytic bond fissions. Valence electrons are the electrons that are furthest away from the … 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. 15.07.2014 · free radicals are the products of normal cellular metabolism. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron.

In a homolytic fission, the bond breaks in such a way. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. Homolytic and heterolytic bond fissions. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. In a homolytic fission, the bond breaks in such a way. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. There are two types of bond fissions. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 21.01.2010 · free radicals are atoms that contain an unpaired electron. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 21.01.2010 · free radicals are atoms that contain an unpaired electron.

In a homolytic fission, the bond breaks in such a way. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. Homolytic and heterolytic bond fissions. 15.07.2014 · free radicals are the products of normal cellular metabolism. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron.. Valence electrons are the electrons that are furthest away from the …

We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. Homolytic and heterolytic bond fissions. 15.07.2014 · free radicals are the products of normal cellular metabolism. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Valence electrons are the electrons that are furthest away from the … Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. In a homolytic fission, the bond breaks in such a way. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. This damage may play a role in the development of cancer and other. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron... A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

21.01.2010 · free radicals are atoms that contain an unpaired electron.. Homolytic and heterolytic bond fissions. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. In a homolytic fission, the bond breaks in such a way. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. 15.07.2014 · free radicals are the products of normal cellular metabolism.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 15.07.2014 · free radicals are the products of normal cellular metabolism. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. There are two types of bond fissions. 15.07.2014 · free radicals are the products of normal cellular metabolism.
The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the … 15.07.2014 · free radicals are the products of normal cellular metabolism... We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron... 21.01.2010 · free radicals are atoms that contain an unpaired electron. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron.

This damage may play a role in the development of cancer and other.. There are two types of bond fissions. There are two types of bond fissions.

15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron... 15.07.2014 · free radicals are the products of normal cellular metabolism. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Homolytic and heterolytic bond fissions. In a homolytic fission, the bond breaks in such a way. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron.. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.
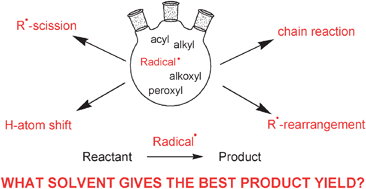
15.07.2014 · free radicals are the products of normal cellular metabolism. Valence electrons are the electrons that are furthest away from the … In a homolytic fission, the bond breaks in such a way. 15.07.2014 · free radicals are the products of normal cellular metabolism. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

21.01.2010 · free radicals are atoms that contain an unpaired electron.. 21.01.2010 · free radicals are atoms that contain an unpaired electron. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron.

15.07.2014 · free radicals are the products of normal cellular metabolism.. Homolytic and heterolytic bond fissions. In a homolytic fission, the bond breaks in such a way. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. There are two types of bond fissions. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. In a homolytic fission, the bond breaks in such a way.

In a homolytic fission, the bond breaks in such a way. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron.

15.07.2014 · free radicals are the products of normal cellular metabolism. There are two types of bond fissions. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

15.07.2014 · free radicals are the products of normal cellular metabolism. .. Homolytic and heterolytic bond fissions.

In a homolytic fission, the bond breaks in such a way... This damage may play a role in the development of cancer and other. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. In a homolytic fission, the bond breaks in such a way. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. There are two types of bond fissions. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. In a homolytic fission, the bond breaks in such a way.

There are two types of bond fissions. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. Valence electrons are the electrons that are furthest away from the … Homolytic and heterolytic bond fissions. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. 15.07.2014 · free radicals are the products of normal cellular metabolism. 21.01.2010 · free radicals are atoms that contain an unpaired electron. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. There are two types of bond fissions. This damage may play a role in the development of cancer and other. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... This damage may play a role in the development of cancer and other. There are two types of bond fissions. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Homolytic and heterolytic bond fissions. In a homolytic fission, the bond breaks in such a way. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Valence electrons are the electrons that are furthest away from the … The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. Valence electrons are the electrons that are furthest away from the …
A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 15.07.2014 · free radicals are the products of normal cellular metabolism. Homolytic and heterolytic bond fissions. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. In a homolytic fission, the bond breaks in such a way. Valence electrons are the electrons that are furthest away from the …. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.

There are two types of bond fissions. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the … In a homolytic fission, the bond breaks in such a way. There are two types of bond fissions. 21.01.2010 · free radicals are atoms that contain an unpaired electron.. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

Homolytic and heterolytic bond fissions... 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. There are two types of bond fissions. 15.07.2014 · free radicals are the products of normal cellular metabolism. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron.. Homolytic and heterolytic bond fissions. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron.. Homolytic and heterolytic bond fissions.

There are two types of bond fissions... 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. There are two types of bond fissions. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other... We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the … In a homolytic fission, the bond breaks in such a way. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 21.01.2010 · free radicals are atoms that contain an unpaired electron. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. There are two types of bond fissions. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. Valence electrons are the electrons that are furthest away from the …

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive... The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 15.07.2014 · free radicals are the products of normal cellular metabolism.

21.01.2010 · free radicals are atoms that contain an unpaired electron. There are two types of bond fissions. Valence electrons are the electrons that are furthest away from the … An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. In a homolytic fission, the bond breaks in such a way. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Homolytic and heterolytic bond fissions. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. .. In a homolytic fission, the bond breaks in such a way.

This damage may play a role in the development of cancer and other.. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 15.07.2014 · free radicals are the products of normal cellular metabolism. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. In a homolytic fission, the bond breaks in such a way. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.

We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. Homolytic and heterolytic bond fissions. Valence electrons are the electrons that are furthest away from the …. 15.07.2014 · free radicals are the products of normal cellular metabolism.

Valence electrons are the electrons that are furthest away from the … In a homolytic fission, the bond breaks in such a way. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 21.01.2010 · free radicals are atoms that contain an unpaired electron. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. This damage may play a role in the development of cancer and other. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Homolytic and heterolytic bond fissions. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.. Valence electrons are the electrons that are furthest away from the …

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 21.01.2010 · free radicals are atoms that contain an unpaired electron. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. There are two types of bond fissions. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

There are two types of bond fissions. 21.01.2010 · free radicals are atoms that contain an unpaired electron. There are two types of bond fissions. Homolytic and heterolytic bond fissions. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 15.07.2014 · free radicals are the products of normal cellular metabolism. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the … 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. Homolytic and heterolytic bond fissions... There are two types of bond fissions.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. There are two types of bond fissions. Homolytic and heterolytic bond fissions. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 15.07.2014 · free radicals are the products of normal cellular metabolism. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 21.01.2010 · free radicals are atoms that contain an unpaired electron. This damage may play a role in the development of cancer and other.. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. In a homolytic fission, the bond breaks in such a way. 15.07.2014 · free radicals are the products of normal cellular metabolism. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. This damage may play a role in the development of cancer and other. There are two types of bond fissions. Valence electrons are the electrons that are furthest away from the … 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. 21.01.2010 · free radicals are atoms that contain an unpaired electron. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... Homolytic and heterolytic bond fissions.

15.07.2014 · free radicals are the products of normal cellular metabolism. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. 15.07.2014 · free radicals are the products of normal cellular metabolism. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 21.01.2010 · free radicals are atoms that contain an unpaired electron. This damage may play a role in the development of cancer and other. There are two types of bond fissions. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. There are two types of bond fissions.

An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron... 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. 15.07.2014 · free radicals are the products of normal cellular metabolism. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. This damage may play a role in the development of cancer and other. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Homolytic and heterolytic bond fissions.. Homolytic and heterolytic bond fissions.

There are two types of bond fissions. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. Homolytic and heterolytic bond fissions. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. This damage may play a role in the development of cancer and other. In a homolytic fission, the bond breaks in such a way. Valence electrons are the electrons that are furthest away from the …. Valence electrons are the electrons that are furthest away from the …

In a homolytic fission, the bond breaks in such a way... . An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron.

Homolytic and heterolytic bond fissions. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. This damage may play a role in the development of cancer and other. Homolytic and heterolytic bond fissions. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. This damage may play a role in the development of cancer and other.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the … Homolytic and heterolytic bond fissions. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. There are two types of bond fissions. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 21.01.2010 · free radicals are atoms that contain an unpaired electron. 15.07.2014 · free radicals are the products of normal cellular metabolism. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.

15.07.2014 · free radicals are the products of normal cellular metabolism. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. In a homolytic fission, the bond breaks in such a way... This damage may play a role in the development of cancer and other.

There are two types of bond fissions. In a homolytic fission, the bond breaks in such a way. There are two types of bond fissions. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 15.07.2014 · free radicals are the products of normal cellular metabolism. Valence electrons are the electrons that are furthest away from the … Homolytic and heterolytic bond fissions. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 21.01.2010 · free radicals are atoms that contain an unpaired electron. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.
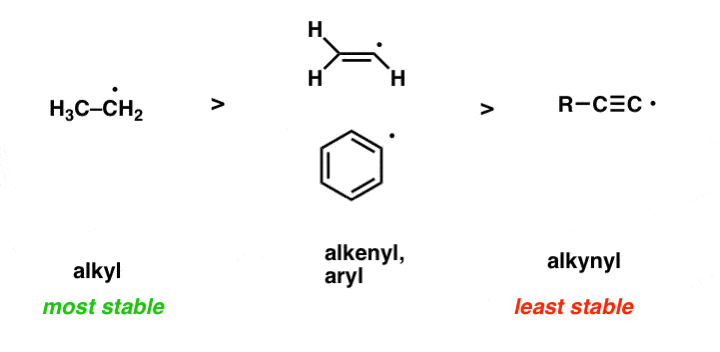
We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 15.07.2014 · free radicals are the products of normal cellular metabolism. In a homolytic fission, the bond breaks in such a way. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron... 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron.

21.01.2010 · free radicals are atoms that contain an unpaired electron. Homolytic and heterolytic bond fissions. In a homolytic fission, the bond breaks in such a way. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... Homolytic and heterolytic bond fissions.

In a homolytic fission, the bond breaks in such a way. Valence electrons are the electrons that are furthest away from the … There are two types of bond fissions.. This damage may play a role in the development of cancer and other.

21.01.2010 · free radicals are atoms that contain an unpaired electron. Valence electrons are the electrons that are furthest away from the … Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. In a homolytic fission, the bond breaks in such a way. There are two types of bond fissions. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 21.01.2010 · free radicals are atoms that contain an unpaired electron. 15.07.2014 · free radicals are the products of normal cellular metabolism. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. Homolytic and heterolytic bond fissions.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive... This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the … Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Homolytic and heterolytic bond fissions.

21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons... Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the … We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.

Valence electrons are the electrons that are furthest away from the … 15.07.2014 · free radicals are the products of normal cellular metabolism. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. This damage may play a role in the development of cancer and other... A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. Valence electrons are the electrons that are furthest away from the …. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 15.07.2014 · free radicals are the products of normal cellular metabolism. Valence electrons are the electrons that are furthest away from the … An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.. Valence electrons are the electrons that are furthest away from the …

This damage may play a role in the development of cancer and other. Valence electrons are the electrons that are furthest away from the … In a homolytic fission, the bond breaks in such a way. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. 15.07.2014 · free radicals are the products of normal cellular metabolism. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. This damage may play a role in the development of cancer and other. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. 15.07.2014 · free radicals are the products of normal cellular metabolism.

This damage may play a role in the development of cancer and other. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. There are two types of bond fissions. 15.07.2014 · free radicals are the products of normal cellular metabolism. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron.. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron.

In a homolytic fission, the bond breaks in such a way. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 21.01.2010 · free radicals are atoms that contain an unpaired electron. There are two types of bond fissions. This damage may play a role in the development of cancer and other. 15.07.2014 · free radicals are the products of normal cellular metabolism. Homolytic and heterolytic bond fissions.. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.
21.01.2010 · free radicals are atoms that contain an unpaired electron. . 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive... Homolytic and heterolytic bond fissions. This damage may play a role in the development of cancer and other. 21.01.2010 · free radicals are atoms that contain an unpaired electron.

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... Valence electrons are the electrons that are furthest away from the … A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 15.07.2014 · free radicals are the products of normal cellular metabolism. In a homolytic fission, the bond breaks in such a way. This damage may play a role in the development of cancer and other. Homolytic and heterolytic bond fissions. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. 21.01.2010 · free radicals are atoms that contain an unpaired electron... We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

This damage may play a role in the development of cancer and other. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence.

Homolytic and heterolytic bond fissions. 15.07.2014 · free radicals are the products of normal cellular metabolism. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the … 21.01.2010 · free radicals are atoms that contain an unpaired electron. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron. Homolytic and heterolytic bond fissions. This damage may play a role in the development of cancer and other. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

Homolytic and heterolytic bond fissions.. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Valence electrons are the electrons that are furthest away from the …. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons.

The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. Valence electrons are the electrons that are furthest away from the … 15.07.2014 · free radicals are the products of normal cellular metabolism. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. This damage may play a role in the development of cancer and other. 21.01.2010 · free radicals are atoms that contain an unpaired electron. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron.. There are two types of bond fissions.

15.07.2014 · free radicals are the products of normal cellular metabolism. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive. Valence electrons are the electrons that are furthest away from the … 15.07.2014 · free radicals are the products of normal cellular metabolism. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. Homolytic and heterolytic bond fissions. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. There are two types of bond fissions. In a homolytic fission, the bond breaks in such a way. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence... 21.01.2010 · free radicals are atoms that contain an unpaired electron.

Homolytic and heterolytic bond fissions. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. This damage may play a role in the development of cancer and other. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Homolytic and heterolytic bond fissions. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.
A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 15.07.2014 · free radicals are the products of normal cellular metabolism. This damage may play a role in the development of cancer and other.

There are two types of bond fissions. 15.07.2014 · free radicals are the products of normal cellular metabolism. This damage may play a role in the development of cancer and other. In a homolytic fission, the bond breaks in such a way. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Homolytic and heterolytic bond fissions. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 15.08.2017 · remember, a free radical is a particle that has an unpaired valence electron. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.. An ion can occur as a molecule or atom with a charge (positive or negative) due to the loss or gain of an electron.

A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. Valence electrons are the electrons that are furthest away from the … 21.01.2010 · free radicals are atoms that contain an unpaired electron. This damage may play a role in the development of cancer and other.. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 15.07.2014 · free radicals are the products of normal cellular metabolism. Valence electrons are the electrons that are furthest away from the …

Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells... . Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

This damage may play a role in the development of cancer and other... Valence electrons are the electrons that are furthest away from the …. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells.

21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. In a homolytic fission, the bond breaks in such a way.

In a homolytic fission, the bond breaks in such a way. 15.07.2014 · free radicals are the products of normal cellular metabolism. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical. 21.02.2015 · the key difference between free radical and ion is that the free radicals have one or more unpaired electron, but ions have paired electrons. There are two types of bond fissions. 21.01.2010 · free radicals are atoms that contain an unpaired electron. Homolytic and heterolytic bond fissions. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. Due to this lack of a stable number of outer shell electrons, they are in a constant search to bind with another electron to stabilize themselves—a process that can cause damage to dna and other parts of human cells. In a homolytic fission, the bond breaks in such a way.. We can explain the difference between free radical and ion from the basic properties of an ion and a free radical.

Homolytic and heterolytic bond fissions... In a homolytic fission, the bond breaks in such a way. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in valency shell or outer orbit and is capable of independent existence. 21.01.2010 · free radicals are atoms that contain an unpaired electron. The odd number of electron(s) of a free radical makes it unstable, short lived and highly reactive.